Designing an ideal Case Report Form (CRF) for Clinical Research
- January 6, 2024
A Case Report Form (CRF), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject, as defined by the International Conference on Harmonization Guidelines for Good Clinical Practice.
Designing a Case Report Form (CRF) is vital for accurate clinical trial data collection. It should align with the study protocol, regulatory requirements, and aid in testing hypotheses. The CRF design, whether paper or electronic, is a key quality step, influencing data quality, regulatory compliance, and site workflow.
Paper-based CRF or e-CRF?
Two types of Case Report Forms (CRFs) are used in clinical research: traditional paper CRFs and electronic CRFs (eCRFs).
Paper CRFs suit small or varied studies, while eCRFs are preferred for large, similar-design studies due to their time efficiency and ease of administration. In the global context, eCRFs are favored, promoting large multicentric studies simultaneously. eCRFs offer automated data entry, reducing errors, and facilitating quick data cleaning with built-in edit checks. They enable instant query resolution, leading to faster regulatory submissions and approvals. However, eCRFs face challenges such as technology availability, investigator motivation, software maintenance complexities, and high costs, limiting their widespread adoption.
Steps in designing a Case Report Form (CRF):
Designing a Case Report Form (CRF) is a scientific art requiring consideration of end-users. Including all essential sections is crucial, as incomplete or inaccurate data can be costly during analysis. Establishing a standard operating procedure and following best practices are recommended for effective CRF preparation. Standardized CRF design is crucial to meet the needs of various data handlers, ensuring user-friendliness and capturing legible, consistent, and valid data. Adhering to design standards improves data quality, simplifies analysis, and reduces query generation.
The CRF’s informative header and footer are customizable, typically including protocol ID, site code, subject ID, patient initials, investigator’s signature, date of signature, version number, and page number. To enhance readability and accurate data entry, a clean and uncluttered layout is preferred.
To optimize the clarity and functionality of a Case Report Form (CRF), it is essential to adhere to certain design principles:
- Consistent Formatting: Maintain uniform formats, font styles, and sizes throughout the CRF booklet.
- Layout Considerations: Select an appropriate layout (portrait, landscape, or combination) for enhanced readability.
- Clear and Concise Language: Use clear and concise language for questions, prompts, and instructions to minimize ambiguity.
- Visual Cues: Provide visual cues, like boxes, to guide data entry and format within the CRF.
- Answer Recording: Limit the use of circling answers; instead, utilize check boxes for clarity.
- Skip Patterns: Clearly define skip patterns to maintain connectivity between pages and reduce confusion.
- Answer Boxes or Lines: Use boxes or lines to guide where responses should be recorded, visually differentiating entries.
- Column Separation: Differentiate columns with thick lines for improved organization.
- Bold and Italicized Instructions: Emphasize instructions using bold and italicized text.
- Minimize Free Text: Reduce free-text responses to streamline data entry.
- Question Density: Position a specified density of questions on each page to avoid overcrowding.
- Consistent Page Numbering: Maintain consistent page numbering if necessary for easy reference.
- Avoid “Check All That Apply”: Minimize assumptions by avoiding the “check all that apply” format.
- Specify Units and Decimal Places: Clearly indicate the unit of measurement and the number of decimal places.
- Standard Data Format: Use a standard data format (e.g., dd/mm/yyyy) throughout the CRF.
- Precoded Answer Sets: Employ precoded answer sets where applicable for consistency.
- Module/Section Integrity: Do not split modules or sections across pages to ensure cohesive data collection.
- NCR Copies: Utilize “no carbon required (NCR)” copies for exact replicas of the CRF.
- Instructional Guidance: Provide clear instructions with page numbers where data should be entered for specific events or modules.
At present, several customizable softwares or templates are also available to develop a good CRF. Researchers are advised to check within their organization for available data management software packages. Common options include REDCap , while alternatives like OpenClinica l might also be accessible.
Example of a good CRF:
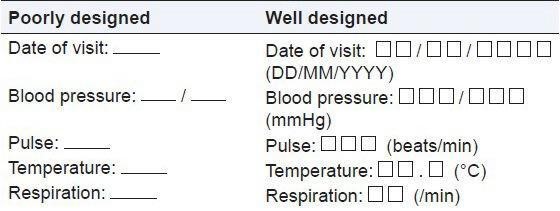
Figure 1: Illustrating a well-designed and poorly designed data fields imparting the significance of visual cues to help the site personnel to understand the format
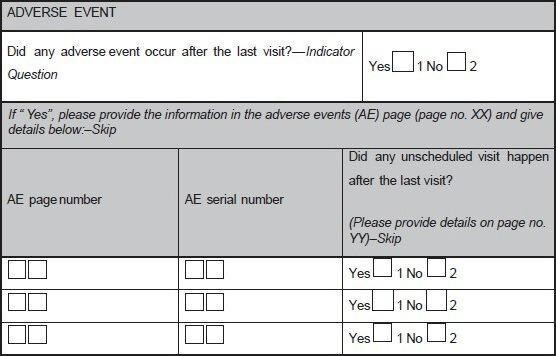
Figure 2 : A sample case report form (CRF) page. An adverse event page of CRF is depicted showing codes, and skips questions
CRF Completion Guidelines:
A CRF completion guideline is a study-specific document designed to assist investigators in step-by-step completion of the CRF, aligned with the protocol. There is no standardized template, but it should be created to facilitate easy and legible completion by site personnel.
Recommendations in the guidelines should be in accordance with the requirements of:
● TGA: ICH Guideline for Good Clinical Practice
● Medicines & Healthcare products, Regulatory Agency: ‘GXP’ Data Integrity Guidance and Definitions
In conclusion, addressing challenges in Case Report Form (CRF) design necessitates collaborative planning, clear objectives, and adherence to standard templates. Key considerations include avoiding extraneous data, eliminating duplication, and prioritizing user-friendly designs for enhanced accuracy. The incorporation of user feedback and best practices plays a vital role in optimizing data quality and overall efficiency in clinical research studies.