Commonly Used Clinical Research Study Designs
- January 15, 2024
Clinical research utilizes diverse study designs to explore medical inquiries. From observational studies to experimental trials, each design offers unique insights. Researchers choose designs based on their specific questions, aiming for reliable and valid results. Understanding these study designs is crucial for advancing medical knowledge, improving treatments, and enhancing healthcare practices.
In epidemiology, clinical study designs are broadly categorized into observational and experimental types. Observational studies, subdivided into descriptive and analytic, generate hypotheses by describing exposure and/or outcomes or measuring associations. Experimental studies, however, test hypotheses through interventions to assess the relationship between exposure and outcome.
1. Observational Study:
Observational studies are clinical research designs, where investigators observe the subjects in their natural settings, without any intervention or manipulation of variables. They focus on gathering data by observing and analysing the behaviour, characteristics, or outcomes of participants.
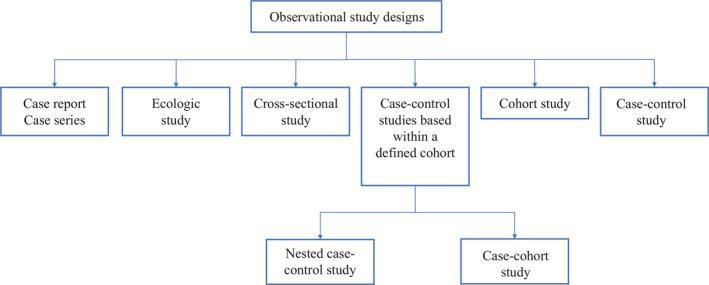
They are further divided into multiple study designs, that includes:
a. Case Reports and Case Series:
Detailed descriptions of atypical clinical cases (case reports) or a group of such cases (case series) provide unique observations, prompting further investigation.
b. Ecologic Study:
Examines population characteristics and identifies correlations between exposures and outcomes at a group level, lacking individual-level associations.
c. Cross-Sectional Study:
Assesses associations between exposures and outcomes at the same time, providing prevalence estimates but lacking temporal associations.
They are commonly used in general population health surveys, and do not establish the directionality of associations between exposure factors and health outcomes. The nature of cross-sectional studies blurs the understanding of the cause-and-effect relationship between diseases and risk factors.
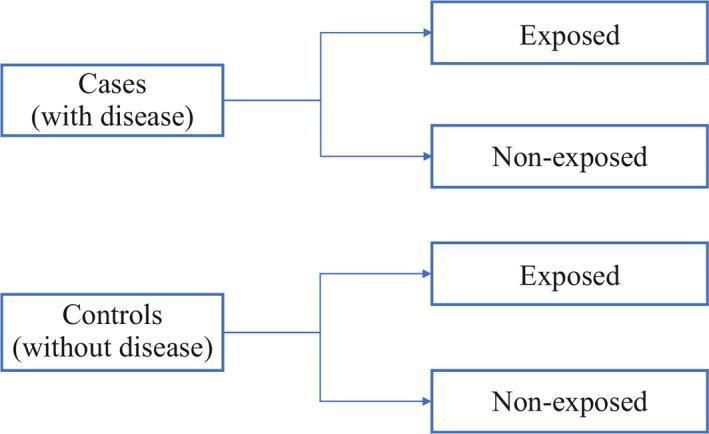
d. Case-Control Study:
Compares groups with and without a disease, exploring risk factors and suggesting causal relationships, yet unable to prove causation.
In a case-control study, researchers create a group of cases (individuals with the outcome of interest) and a similar group of controls (without the outcome). Analyzing historical factors, they identify if certain exposures are more common in cases than controls. If an exposure is more prevalent among cases, researchers may hypothesize a potential link to the studied outcome.
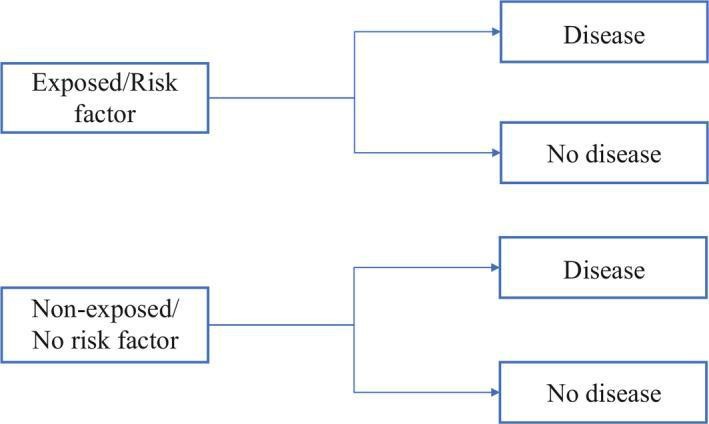
e. Cohort Study:
Cohort studies compare groups with and without a specific exposure to evaluate disease incidence, allowing inference of a causal relationship but not definitive establishment.
Cohort studies, categorized as prospective and retrospective, are selected when the suspected exposure is known and rare, with anticipated high disease incidence in the exposed group. The decision between prospective and retrospective design hinges on the accuracy and reliability of past records regarding the exposure or risk factor.
f. Case-control studies within a defined cohort:
It combines some of the features of a cohort study design and a case‐control study design, baseline information collected before disease onset eliminates recall bias.
g. Nested Case-Control:
Matches cases and controls based on calendar time and follow-up length, with potential for controls becoming cases.
h. Case-Cohort:
Uses a defined sub-cohort for controls, not matched on time, enabling comparisons across different disease groups within the same sub-cohort.
2. Experimental Study Design:
Experimental study design aims to assess the impact of an intervention, where the investigator controls the exposure or treatment. It is a hypothesis testing approach that provides strong evidence for causality. Experimental studies can be categorized as controlled (with a comparison group) or uncontrolled (without a comparison group), each influencing the validity of study conclusions.
Experimental study design is further categorized to the following:
a. Clinical Trials:
Clinical trials, also termed therapeutic trials, involve subjects with a disease who are assigned to different treatment groups.
Clinical trials, seeking generalizability, select representative samples and define key endpoints, such as continuous, ordinal, rates, or time-to-event outcomes. Trial designs include randomized, non-randomized, cross-over, and factorial approaches.
- Randomized Clinical Trials:
Randomized Clinical Trials (RCTs) randomize subjects into intervention/experimental and control groups, minimizing bias for robust outcomes and intervention efficacy assessment, but ethical considerations limit use in rare and serious diseases.
- Non-randomized Clinical Trials
Non-randomized trials lack randomization, relying on predictable patterns for control selection. Historically controlled studies use past data for cost-effectiveness but may lack accuracy and completeness, posing design disadvantages.
- Cross-Over Clinical Trials:
Two groups undergo the same intervention at different times, acting as each other’s control. A ‘washout’ period is advised to eliminate residual effects, suitable when outcomes can be reversed.
- Factorial Trials:
Used to test two independent drugs on the same population, dividing it into four groups – drug A, drug B, both, and neither. This design saves time, but isn’t suitable if the drugs overlap in action or effects.
b. Community Trials:
Also known as cluster-randomized trials, they assign groups in a specific area to different interventions. This provides large-scale results but may not capture individual variability.
c. Field Trials:
Field trials, or preventive trials, assign disease-free subjects to interventions (e.g., vitamins) and monitor them for the occurrence of a specific disease over time.
References:
1. Chidambaram AG, Josephson M. Clinical research study designs: The essentials. PediatrInvestig.2019 Dec 21;3(4):245-252. doi: 10.1002/ped4.12166. PMID: 32851330; PMCID: PMC7331444.
2. Goldberg RJ, McManus DD, Allison J. Greater knowledge and appreciation of commonly-used research study designs. Am J Med. 2013 Feb;126(2):169.e1-8. doi: 10.1016/j.amjmed.2012.09.011. PMID: 23331447; PMCID: PMC3553494.